Answer:

Step-by-step explanation:
There are three heat transfers involved.
heat from combustion of propane + heat gained by water + heat gained by calorimeter = 0
q₁ + q₂ + q₃ = 0
m₁ΔH + m₂C₂ΔT + C_calΔT = 0
Data:
m₁ = 2.1 g
m₂ = 280 g
Ti = 25.00 °C
T_f = 26.55 °C
Ccal = 92.3 J·°C⁻¹
Calculations:
Let's calculate the heats separately.
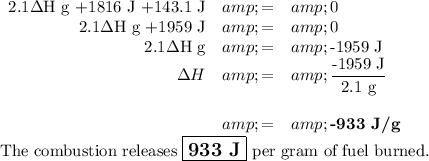
1. q₁
q₁ = 2.1 g × ΔH = 2.1ΔH g
2. q₂
ΔT = T_f - Ti = 26.55 °C - 25.00 °C = 1.55 °C
q₂ = 280 g × 4.184 J·°C⁻¹ × 1.55 °C = 1816 J
3. q₃
q₃ = 92.3 J·°C⁻¹ × 1.55 °C = 143.1 J
4. ΔH