Answer:
Ethanol.
Step-by-step explanation:
Hello!
In this case, since the Fischer esterification stand for the organic acid-base reactions, we infer that the alcohol is related to the -yl part on the name (ethyl propanoate) as the alcohol is likely to lose the -OH and provide a carbocation for the carboxyl group of the carboxylic acid.
In such a way, since the -yl part is given as ethyl, we infer the used alcohol is ethanol:
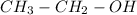
Best regards!