Answer:
Yes, it can can be completed adiabatically
Step-by-step explanation:
To solve the problem we will resort to the theory of thermodynamics,
It is necessary to develop this problem to resort to the A-11E tables in English Units for R134a (since the problem requires it, if it were SI just to change to that table)
State 1 indicates that the refrigerant is at 60 ° F,
In the first table (attached image of the value taken) the value of the entropy is
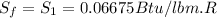
For State 2 the refrigerant is at 50% quality and at a pressure of
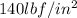
In table 2 of the refrigerant (for the pressure values) we perform the reading and we have to
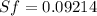
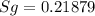
We know that,
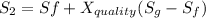
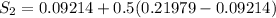
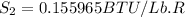
The change in enthalpy would be given by
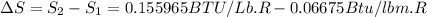
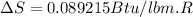
The change in enthalpy is positive, so the process can be completed adiabatically