Answer: The ratio of number of hydroxide ions to iodide ions is 2 : 1.
Step-by-step explanation:
The chemical equation for the oxidation of iodide ion is oxidized to hypoiodite ion by permanganate follows:
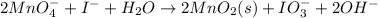
According to mole concept:
1 mole of a substance contains
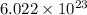 number of particles
number of particles
Moles of iodide ions = 1 mole
Number of iodide ions =
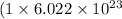
Moles of hydroxide ions = 2 mole
Number of hydroxide ions =
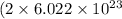
Taking the ratio of number of hydroxide ions and number of iodide ions are as follows:
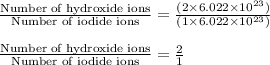
Hence, the ratio of number of hydroxide ions to iodide ions is 2 : 1.