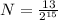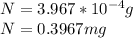Answer:
0.3967mg of sodium-24 are left
Step-by-step explanation:
If the half life of radioactive element (which is sodium-24 here) is
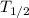 , then at the end of this time the number of atoms in the sample will become half i.e. 1/2 .
, then at the end of this time the number of atoms in the sample will become half i.e. 1/2 .
if the original amount of atoms is N°. The formula for calculating the remaining atoms (N) left in the sample after t half lives would be:
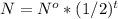
Here, in this question,
Given amount of sodium-24 = N° = 13 grams
Half life of sodium-24 = t = 15
and Remaining amount of sodium after 45 hours = N = ?
Therefore, using the given Forumla: