Answer:
1)2676.5kJ/KG
2)57.87MW
Step-by-step explanation:
Hello!
To solve this problem use the following steps
1. We will call state 1 at the entrance of the boiler and 2 at the exit.
2. use thermodynamic tables to find enthalpy enthalpies in states 1 and 2, and density in the state 2
note:Through laboratory tests, thermodynamic tables were developed, these allow to know all the thermodynamic properties of a substance (entropy, enthalpy, pressure, specific volume, internal energy etc ..)
through prior knowledge of two other properties such as pressure and temperature.
h1=Enthalpy(Water;T=24C;P=1000kPa) =101.5kJ/kg
h2=Enthalpy(Water;x=1(quality);P=1000kPa)=2778kJ/kg
density2(Water;x=1(quality);P=1000kPa)=5.144Kg/m^3
3.find the difference between the two enthalpies to know ΔH (in kJ/kg)
ΔH=h2-h1=2778-101.5=2676.5kJ/KG
4. Find the mass flow by multiplying the volumetric flow in state 2 and the density in state 2
m=mass flow=(5.144kg/m^3)(15,000 m 3 /h)=77160kg/h=21.4kg/s
5.Find the flow rate by dividing the volumetric flow (15000m ^ 3 / h = 4.167m ^ 3 / s) between the cross-sectional area of the pipe
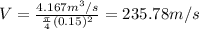
6. use the first law of thermodynamics in the boiler, it is established that the energy that enters a system is the same that must leave, take into account flow energies, kinetic energy and added heat
mh1+Q=mh2+0.5mV^2
solving for Q
Q=m(h2-h1)+0.5mV^2
Q=21.4(2778000-101500)+0.5(21.4)(235.789)^2=57871982w=57.87Mw