Answer:
A. 7.2%
B. 8.6 ppm
Step-by-step explanation:
Part A
First let's calculate the mass of I₂, using the known value of moles and the molecular weight (253.8 g/mol)
- 4.0x10⁻² mol I₂ * 253.8 g/mol = 10.152 g I₂
Mass percentage is calculated using the mass of I₂ and the total mass (mass of I₂ + mass of CCl₄)
- Total mass = 130 + 10.152 = 140.152 g
- Mass percentage I₂ = 10.152 / 140.152 * 100 = 7.2%
Part B
The concentration of Sr⁺² in ppm is calculated using the formula
We're given the mass of Sr⁺² in grams, so now we convert it into mg:
- 8.2x10⁻³g *
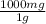 = 8.2 mg
= 8.2 mg
Now to convert kg of water into L, we use the density of seawater (1050 kg/m³):
Converting density: 1050
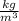 *
*
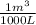 = 1.05 kg/L
= 1.05 kg/L
- Volume of one kilogram of water = 1kgWater ÷ 1.05 kg/L = 0.95 L
Finally we calculate the concentration of Sr⁺²:
- 8.2 mg / 0.95 L = 8.6 ppm