Answer:
Following are the solution to the given question:
Step-by-step explanation:
Let us indicate H3AsO4, an H3A triprotic acid; the ionising equations are as follows:
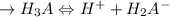
![\to Ka_1 = ([H^+][H_2A^-])/([H_3A])](https://img.qammunity.org/2022/formulas/chemistry/college/mar5w8sf5he810xubpb6nqx9152gs2bt0c.png)
![= 10-2.2 = 6.31 * 10^(-3) \ \ \ \ \ \ \ \ [since\ pKa_1 = 2.2\ and \ Ka = -\log_(10)(pKa)]\\](https://img.qammunity.org/2022/formulas/chemistry/college/c189184c855ua1uo6yzpyuegtpltvwamv7.png)
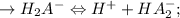
![\to Ka_2 = ([H^+][HA_2^-])/([H_2A^-]) = 10-6.8 = 1.58 * 10^(-7) \\(since pKa2 = 6.8)\\\\\to HA_2^- \Leftrightarrow H^+ + A_3^- ;\\\\ \to Ka_3 = ([H^+][A_3^-])/([HA_2^-]) = 10-11.6 = 2.51 * 10^(-12) \\ (given pKa_3 = 11.6)](https://img.qammunity.org/2022/formulas/chemistry/college/qoe3niikzlpwy7sfkbcpvcnz69e1gbg248.png)
The initial pH; we've 0.00 mL of 0.100 M NaOH – H3A is the main species; Ka1>>Ka2>>Ka3 is also noted. They therefore ignore H2A's disconnection but set up next ICE chart.
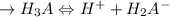
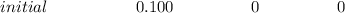
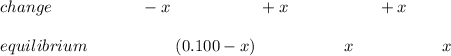
![Ka_1 = ([H^+][H_2A^-])/([H_3A]) = ((x)(x))/((0.100 - x))](https://img.qammunity.org/2022/formulas/chemistry/college/jmauptt9poo1ap4do6k44gjod524zbtkyi.png)
![\text{x to be much smaller than 0.100 M}\\\\to 6.31 * 10^(-3) = (x^2)/(0.100)\\\\\to x^2 = 6.31 * 10^(-4)\\\\ \to x = 0.025\ M\\\\\to pH = -\log_(10)[H^+] = -\log_(10)(0.025) = 1.60](https://img.qammunity.org/2022/formulas/chemistry/college/unl7kkvwmsweb75vcwq9nksv7sttv6zstn.png)