Answer:
c) Kinetic energy
Step-by-step explanation:
By kinetic mole theory of gas an equation is derived saying
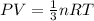
and by ideal gas equation we know,
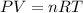
By combining these equations we get,
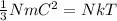
And that equation can be rearranged as,
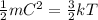
So we find that ,
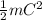 = Kinetic energy of a gas molecule.
= Kinetic energy of a gas molecule.
So as temperature increase kinetic energy of molecules increase.
Please consider that : C² = root mean square velocity
All other symbols in usual notation.
Although ideal gas equations cannot be directly applied to real gases, the behavior of real gases is similar but not as exactly according to the ideal gas equations.