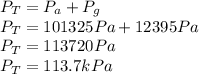The pressure value is given by the equation,
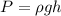
Where,
 represents the density of the liquid
represents the density of the liquid
g= gravity
h= Heigth
A) For the measurement of the guage pressure we have the data data,
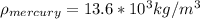
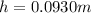
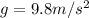
Replacing we get,
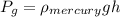
![[tex]P_g = (13.6*10^3)(9.8)(0.0930)](https://img.qammunity.org/2020/formulas/physics/college/tahozmlc5q51ot3p67cxk4evi1rvjiego1.png) P_g = 12395Pa[/tex]
P_g = 12395Pa[/tex]
In order to find the Absolute pressure, we perform a sum between the atmospheric pressure and that of the Gauge,
B) The atmospheric pressure at sea level is 101325Pa, assuming ideal conditions, we will take this pressure for our calculation, so