Answer:
D. 1s2,2s2,2p6,3s2,3p6,3d10,4s2,4p6,4d10,5s2,5p6
Step-by-step explanation:
Hello!
In this case, since the standard iodine atom has 53 electrons, when it forms the iodide ion it is known it gains one spare electron so now it has 54; it means we need to write the new electron configuration up to 54 as shown below:
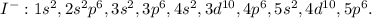
Thus, the answer should be:
D. 1s2,2s2,2p6,3s2,3p6,3d10,4s2,4p6,4d10,5s2,5p6
Even when the order is not the adequate one.
Regards!