Answer :
(a) The final temperature is
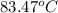
(b) This is not possible because the final temperature can not be more than the starting temperature of the coffee.
Explanation :
(a) Heat gained by the Al = Heat lost by the coffee
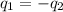
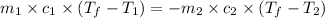
where,
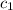 = specific heat of Al =
= specific heat of Al =
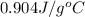
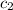 = specific heat of coffee =
= specific heat of coffee =
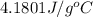
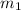 = mass of Al = 36 g
= mass of Al = 36 g
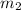 = mass of coffee = 180 g
= mass of coffee = 180 g
 = final temperature = ?
= final temperature = ?
 = initial temperature of Al =
= initial temperature of Al =
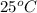
 = initial temperature of coffee =
= initial temperature of coffee =
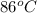
Now put all the given values in the above formula, we get
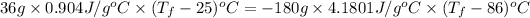
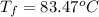
Thus, the final temperature is
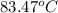
(b) As per question, the first time a student solved this problem she got an answer of 89°C that means the final temperature is higher than the starting temperature of the coffee.
So, this is not possible because the final temperature can not be more than the starting temperature of the coffee.