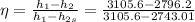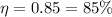Our values are given by,
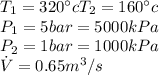
From steam tables A5 we can calculate the values for State 1, that is,

Here is easily to find the flow mass rate, through
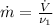
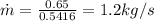
We apply a similar process to State 2, then

With this values we can now calculate the Power, which is given by,
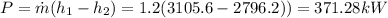
The total rate in entropy, that is
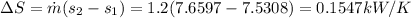
To find the efficiency we know that in a isentropic state, the entropy
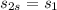 then
then
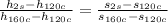
Replacing to find
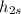
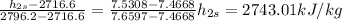
Then the efficiency is given by,