Answer:
1. To recover he material completely the best way is to evaporate the solvent and try to recrystallize it once more. Or you can add less polar solvent or that which wont dissolve your compound to precipitate it.
2. The density of methyl salicylate is 1.174 g/cm3 so 1.3 mL is 1.3/1.174=1.107 g so in theory after hydrolysis you are supposed to obtain
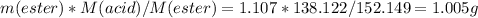 so the yield will be
so the yield will be
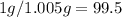
3. The best variant is when your compound is practically insoluble in cold solvent and dissolves fairly in hot solvent (example sodium acetate in water). The slower the crystals grow, the better for their purity. If compound crystallizes fast occlusion can take place and you won't get pure crystals but that ones with solvent inside and impurities.
Step-by-step explanation: