Answer: Option (D) is the correct answer.
Step-by-step explanation:
Metals are the species which tend to lose electrons in order to attain stability. When a metal loses one electron then it acquires a +1 charge and if a metal loses 2 electrons then it acquires a +2 charge and so on.
On the other hand, non-metals are the species which tend to gain electrons in order to attain stability.
When one electron is gained by a non-metal then it acquires a -1 charge and when two electrons are gained by a non-metal then it acquires a -2 charge and so on.
For example, a metal of group 2 will have a +2 charge in ionic form and when it combines with a non metal who needs to gain one or two electrons then the compound formed will be
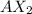 .
.
As
 can be assumed in place of
can be assumed in place of
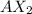 .
.
Thus, we can conclude that the statement A is a metal from group 2, and X is a nonmetal from group 7.