Answer : The mass of
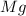 required is 30.38 mg.
required is 30.38 mg.
Explanation :
To calculate the moles of hydrogen gas, we use the equation given by ideal gas :
PV = nRT
where,
P = Pressure of hydrogen gas = 754 torr
V = Volume of the hydrogen gas = 31.2 mL = 0.0312 L
n = number of moles of hydrogen gas = ?
R = Gas constant =
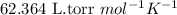
T = Temperature of hydrogen gas =
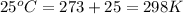
Putting values in above equation, we get:
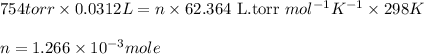
Now we have to calculate the moles of
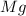 .
.
The balanced chemical reaction is:
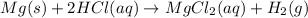
From the balanced chemical reaction, we conclude that
As, 1 mole of
 produced from 1 mole
produced from 1 mole
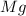
As,
 mole of
mole of
 produced from
produced from
 mole
mole
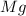
Now we have to calculate the mass of
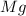 .
.
Molar mass of
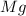 = 24 g/mol
= 24 g/mol
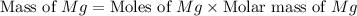
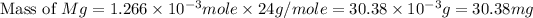
conversion used : (1 g = 1000 mg)
Therefore, the mass of
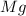 required is 30.38 mg
required is 30.38 mg