Answer:
-11.4 kJ of heat are released
Step-by-step explanation:
Balanced reaction-

According to balanced equation, 1 mol of HCl is neutralized by 1 mol of NaOH.
So, 200 mL of 1 M HCl will be completely neutralized by 200 mL of 1 M NaOH.
Let's assume both HCl and NaOH solutions have densities equal to density of pure water.
We know, density = (mass)/(volume)
Density of pure water = 1 g/mL
So mass of both solutions =
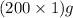 = 200 g
= 200 g
So, mass of mixture = (200+200) g = 400 g
Hence, Amount of heat released =
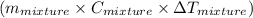
Where, m represents mass, C represents heat capacity and
 represents change in temperature
represents change in temperature
Let's assume that heat capacity of mixture is also equal to heat capacity of water.
Heat capacity of water = 4.186 J/(g.
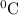 )
)
So, amount of heat released =
![[400g* 4.186J.g^(-1).^(0)\textrm{C}^(-1)*(27.8-21.0)^(0)\textrm{C} ]](https://img.qammunity.org/2020/formulas/chemistry/high-school/ax93d7jwjelf7wklbom8kd4toxywpg6u5q.png) =
=
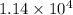 J=11.4 kJ
J=11.4 kJ
As acid-base neutralization reactions are exothermic therefore-
Heat released = -11.4 kJ