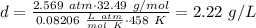Answer:
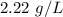
Step-by-step explanation:
According to the ideal gas law equation:
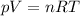
Let's express the number of moles in terms of mass and molar mass:
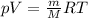
Let's divide both sides by volume:
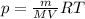
Notice that:
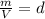
So the equation becomes:
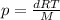
Expressing density:
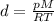
Substituting the given values: