Answer:
Volume of HCl = 0.0688 L
Step-by-step explanation:
pH is defined as the negative logarithm of the concentration of hydrogen ions.
Thus,
pH = - log [H⁺]
So, pH = 1.3
Thus, 1.3 = - log [H⁺]
[H⁺] = 0.05012 M
Since, HCl is a strong acid, the concentration of HCl = 0.05012 M
Also,
Moles of
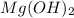 :-
:-
Mass =
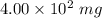
Also, 1 mg = 0.001 g
So =, Mass =
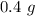
Molar mass of
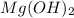 = 58.32 g/mol
= 58.32 g/mol
The formula for the calculation of moles is shown below:

Thus,
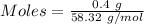
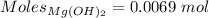
According to the reaction:-
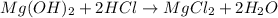
1 mole of
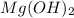 neutralizes 2 moles of HCl
neutralizes 2 moles of HCl
Considering:

So,
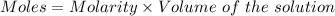
Thus,
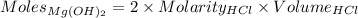
Appyling values as:-
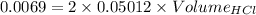
Volume of HCl = 0.0688 L