Answer: 0.5 moles are present in 17.0 g of
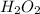 .
.
Step-by-step explanation:
Number of moles present in a substance is the given mass divided by molar mass of the substance.
As the given mass of
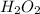 is 17.0 g and its molar mass is 34 g/mol.
is 17.0 g and its molar mass is 34 g/mol.
Hence, number of moles present in given 17.0 g of
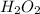 is as follows.
is as follows.
No. of moles =
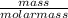
=
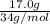
= 0.5 moles
Therefore, 0.5 moles are present in 17.0 g of
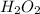 .
.