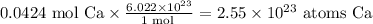There are
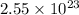 atoms in 1.7 mol Ca
atoms in 1.7 mol Ca
Solution:
Initially we have to convert the given mass of calcium to moles of calcium, using its molar mass (referring to a periodic table, this is 40.08 g/mol
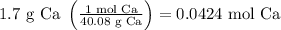
Using Avogadro's number,
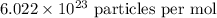 as
as
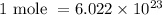
We can calculate the number of atoms present by