Answer:
Both chlorine
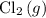 and hydrogen chloride
and hydrogen chloride
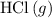 .
.
Concentrated sulfuric acid should not be used to dry ammonia because ammonia itself would react with sulfuric acid.
Step-by-step explanation:
Keep in mind that concentrated sulfuric acid is:
- Acidic, and
- Capable of oxidizing many substances.
Because of its acidity, concentrated sulfuric acid will readily react with gases that form basic solutions when mixed with water. Therefore, concentrated sulfuric acid should not be used to dry those gases. Neither should concentrated sulfuric acid be used to dry gases that might lose electrons to concentrated sulfuric acid. Otherwise, a significant portion of those gas would be consumed in those unexpected chemical reactions.
Ammonia
Ammonia is one such gas. Because the solubility of ammonia in water is remarkably high, the humid ammonia gas in this question would likely exist in the form
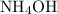 . When that gas comes into contact with sulfuric acid
. When that gas comes into contact with sulfuric acid
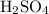 , the following reaction could take place:
, the following reaction could take place:
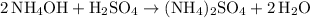 .
.
As long as concentrated sulfuric acid isn't used up, very little ammonia would exit from the other side of the drying apparatus. Hence, concentrated sulfuric acid shouldn't be used to dry ammonia.
Chlorine and HCl
Concentrated sulfuric acid does not react with chlorine or HCl under typical lab conditions. Therefore, it should be possible to dry chlorine
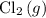 and
and
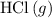 with concentrated sulfuric acid in a lab.
with concentrated sulfuric acid in a lab.