Answer:
Molarity=0.0058M
Step-by-step explanation:
Given the weight of AgN
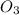 is(w) 1.24g
is(w) 1.24g
We know that the molecular weight of AgN
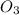 is(W) 169.87g/mol.
is(W) 169.87g/mol.
Given the volume of the solution is(V) 125ml.
Here solute is silver nitrate and the solvent is water.
We know that
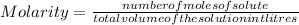
We know that number of moles=weight/molecular weight
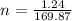 =
=
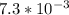
volume of the solution(V)=125/1000=0.125L
Now
Molarity=n/V
M=
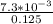 =0.00584M
=0.00584M