Answer:
Temperature is always directly proportional to average kinetic energy of the material.
Step-by-step explanation:
The average kinetic energy is given by the formula
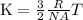
Where K = average kinetic energy . R = gas constant , NA = avogadro's number, T = temperature
From the formula we can clearly see that K is directly proportional to T i.e kinetic energy increases with increase in temperature.
As a substance when it absorbs the heat , temperature increases which makes the faster movement of the particles thus increasing the kinetic energy.The above formula is applicable to gaseous molecules.