Answer :
The correct expression for rate of reaction is:
![Rate=-(d[A])/(dt)=-(1)/(2)(d[B])/(dt)=+(d[C])/(dt)](https://img.qammunity.org/2020/formulas/physics/college/nvdkqzirgn10sa9cg6xfo2ec0b0noqkzgz.png)
The [A] decreasing at 0.14 mol/L.s
Explanation :
The given rate of reaction is,
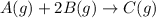
The expression for rate of reaction :
![\text{Rate of disappearance of A}=-(d[A])/(dt)](https://img.qammunity.org/2020/formulas/physics/college/3zikihbhpabiiijtu4muze2ckjexl37pu4.png)
![\text{Rate of disappearance of B}=-(1)/(2)(d[B])/(dt)](https://img.qammunity.org/2020/formulas/physics/college/b64iezdti4u7qff65gnlmzzr3tf7ewq2n6.png)
![\text{Rate of formation of C}=+(d[C])/(dt)](https://img.qammunity.org/2020/formulas/physics/college/6gep8hobrokfvv14yhjel9sff8fwqp0ywa.png)
So,
![Rate=-(d[A])/(dt)=-(1)/(2)(d[B])/(dt)=+(d[C])/(dt)](https://img.qammunity.org/2020/formulas/physics/college/nvdkqzirgn10sa9cg6xfo2ec0b0noqkzgz.png)
Now we have to calculate how fast is [A] decreasing.
Given:
![-(d[B])/(dt)=0.28mol/L.s](https://img.qammunity.org/2020/formulas/physics/college/6o7p05dus072snbvsx5b48zfvqpwrwk8vp.png)
![-(1)/(2)(d[B])/(dt)=-(1)/(2)* 0.28mol/L.s=-0.14mol/L.s](https://img.qammunity.org/2020/formulas/physics/college/rzz918shvcajx8oh4cdw3cvznbwdc8vvu1.png)
![-(d[A])/(dt)=-(1)/(2)(d[B])/(dt)=-0.14mol/L.s](https://img.qammunity.org/2020/formulas/physics/college/fkjwshwsdbayfyzn121xlp21n24wl05bnb.png)
Hence, the [A] decreasing at 0.14 mol/L.s