Answer:
1.45 kilo joules of heat is produced when 100 mL of 0.250 M HCl and 200 mL of 0.150 M NaOH.
1.15°C is the temperature increase.
Explanation:
 ,ΔH°=-58 kJ/mol
,ΔH°=-58 kJ/mol
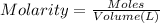
Molarity of HCl = 0.250 M
Volume of HCl = 100 ml = 0.1 L
Moles of HCl = n
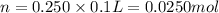
Molarity of NaOH= 0.150 M
Volume of NaOH= 200 ml = 0.2 L
Moles of NaOH= n'
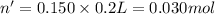
According to reaction, 1 mol of HCl reacts with 1 mol of NaOH. Then 0.0250 mole of HCl will reacts with 0.0250 mol of NaOH.
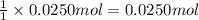 of NaOH
of NaOH
As we can see that moles of NaOH are in excess.Hence, excessive agent.
The enthalpy of the reaction = ΔH°=-58 kJ/mol
Energy released when 0.0250 moles of HCl reacted with 0.0250 moles of NaOH:
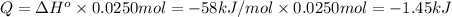
(Negative sign indicates that heat is liberated.)
Mass of the HCL solution = m
Volume of HCl ,v= 100 ml
Density of HCl solution = d = 1.00 g/mL
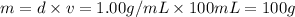
Mass of NaOH solution = m'
Volume of NaOH ,v' = 200 ml
Density of NaOH solution = d' = 1.00 g/mL
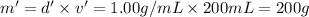
Mass of the solution after mixing,M = m + m' = 100 g + 200 g = 300 g
Heat absorbed by the final solution formed after mixing = Q'
Heat absorbed by the final solution formed after mixing = Heat released during reaction
Q' = -Q = -(-1.45 kJ)= 1.45 kJ=1450 J (1kJ = 1000 J)
Q' = mcΔT
generally, m = mass of the substance
c = specific heat of the substance
ΔT = Change in temperature
Specific heat capacity of the product formed after mixing = c = 4.19 J/g°C
Mass of the resulting mix = 300 g
ΔT = ?

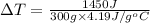
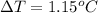
1.15°C is the temperature increase.