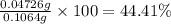Answer:
Percentage of an aldrin in the sample is 44.41%.
Step-by-step explanation:
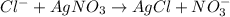
Molarity of the silver nitrate solution = 0.03337 M
Volume of the silver nitrate = 23.28 mL = 0.02328 L
Moles of silver nitrate = n
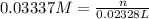
n =
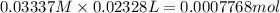
According to reaction 1 mole of silver nitrate recats with 1 moles of chloride ions.
Then 0.0007768 moles of silver nitrate will react with:
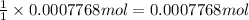 chloride ions.
chloride ions.
In one mole of aldrin there are 6 moles of chloride ions.
Then moles of aldrin containing 0.0007768 moles chloride ions are:
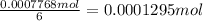
Moles of aldrin present in the sample = 0.0001295 mol
Mass of 0.0001295 moles of aldrin present in the sample :
0.0001295 mol × 364.92 g/mol =0.04726 g
Percentage of an aldrin in the sample: