Answer:
Lead shows the greatest temperature change upon absorbing 100.0 J of heat.
Step-by-step explanation:
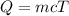
Q = Energy gained or lost by the substance
m = mass of the substance
c = specific heat of the substance
ΔT = change in temperature
1) 10.0 g of copper
Q = 100.0 J (positive means that heat is gained)
m = 10.0 g
Specific heat of the copper = c = 0.385 J/g°C
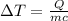
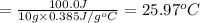
2) 10.0 g of aluminium
Q = 100.0 J (positive means that heat is gained)
m = 10.0 g
Specific heat of the aluminium= c = 0.903 J/g°C
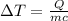
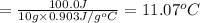
3) 10.0 g of ethanol
Q = 100.0 J (positive means that heat is gained)
m = 10.0 g
Specific heat of the ethanol= c = 2.42 J/g°C
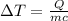
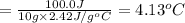
4) 10.0 g of water
Q = 100.0 J (positive means that heat is gained)
m = 10.0 g
Specific heat of the water = c = 4.18J/g°C
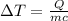
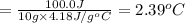
5) 10.0 g of lead
Q = 100.0 J (positive means that heat is gained)
m = 10.0 g
Specific heat of the lead= c = 0.128 J/g°C
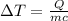
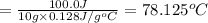
Lead shows the greatest temperature change upon absorbing 100.0 J of heat.