Answer:
9.10 atm
Step-by-step explanation:
At the normal boiling point, the vapor pressure of a liquid is equal to the atmospheric pressure, 1 atm. Because ΔHvap doesn't change, we can use the Clausius-Clayperon equation to determinate the pressure at the other temperature.
So, for p1 = 1 atm, T1 = -29.2ºC + 273 = 243.8 K, T2 = 40ºC + 273 = 313 K, R = 8.3145 J/molK (gas constant):
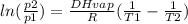
ln(p2/1) = (20250/8.3145)*(1/243.8 - 1/313)
ln(p2) = 2435.50424*9.068x10⁻⁴
ln(p2) = 2.2086
p2 =
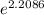
p2 = 9.10 atm