Answer:
The answer to your question is: 81%
Step-by-step explanation:
Data
CaO = 2 g
Ca(OH)₂ = 2.14 g
Reaction balanced
CaO + H₂O ⇒ Ca(OH)₂
Molecular mass
Calcium oxyde = 40 + 16 = 56 g
Calcium hydroxide = 40 + 2 + 32 = 74 g
Process
Rule of three
56 g of CaO -------------- 74 g of Ca(OH)₂
2 g of CaO ------------- x
x = (2 x 74) / 56
x = 2.64 g of Ca(OH)₂
Percent yield
% Yield =
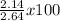
% yield = 81