Step-by-step explanation:
A chemical reaction where two chemical compounds exchange ions with each other is called a double displacement reaction.
Also, when an insoluble salt is formed due to chemical reaction between two chemical compounds is called a precipitation reaction.
For example,
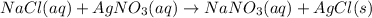
Here, ions are exchanged between the compounds
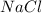 and
and
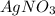 . Also,
. Also,
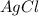 is the precipitate formed.
is the precipitate formed.