Step-by-step explanation:
When water is heated up then its molecules move rapidly from one place to another. Hence, kinetic energy of the molecules tend to increase.
Also, K.E =
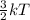
where, T = temperature
Therefore, more is the increase in temperature more will be the mean molecular speed change in the water molecules.
As a result, mean molecular speed change increases with increase in temperature and vice-versa.