Answer:
The ratio of the translational rms speed in the ionosphere to the translational rms near the earth's surface is
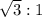
Step-by-step explanation:
The relation between the translational rms speed and the temperature is given by :
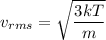
So,
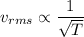
When the temperature is three times greater.
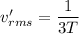
The ratio of the translational rms speed in the ionosphere to the translational rms near the earth's surface is :
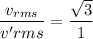
Hence, this is the required solution.