Newly occupied volume is 7.672L by N2 gas.
Explanation:-
Given
V= 9.20 L, T(i) = T (f) = 294 K, P (i) = 0.959 atm, P (f) = 1.15 atm
To Find V (f)
Solution
According to the gas equation
PV= nRT
Where P=Pressure
V=Volume
n=No of moles
R=Universal gas constant
T=Temperature
Also we know the relation between pressure, volume and temperature as
PV/T = constant
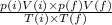
![V(f)=[P(i) V(i) T(f)] /[T(i) P(f)]](https://img.qammunity.org/2020/formulas/chemistry/middle-school/8s4lp0t3fpf63jr2hn0wyvaqld56beihq9.png)
Where P(i)V(i)/T(i) are the initial values of Pressure, Volume and Temperature.
P(f)V(f)/T(f) are the final values of Pressure, Temperature and Volume.
Substitute the known values in the equation we get
=
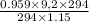
=
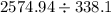
= 7.672 L
Thus the final volume of the system is 7.672 L