Answer:
87.40 %
Step-by-step explanation:
Concept being tested: Percent yield of a product
We are given;
Mass of Sodium oxide 5 g
Experimental or Actual yield of sodium peroxide IS 5.5 g
We are required to calculate the percent yield of sodium peroxide;
The equation for the reaction that forms sodium peroxide is
2Na₂O + O₂ → 2Na₂O₂
Step 1; moles of sodium oxide
Moles = mass ÷ molar mass
Molar mass of sodium oxide is 61.98 g/mol
Therefore;
Moles = 5 g ÷ 61.98 g/mol
= 0.0807 moles
Step 2: Theoretical moles of sodium peroxide produced
From the equation, 2 moles of sodium oxide produces 1 mole of sodium peroxide.
Thus, moles of sodium peroxide used is 0.0807 moles
Step 3: Theoretical mass of sodium peroxide used
Mass = Number of moles × Molar mass
Molar mass of sodium peroxide = 77.98 g/mol
Therefore;
Theoretical mass = 0.0807 moles × 77.98 g/mol
= 6.293 g
Theoretical mass of Na₂O₂ is 6.293 g
Step 4: Percent yield of Na₂O₂
- We know that percent yield is given by the ratio of actual yield to theoretical yield expressed as a percentage.
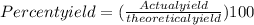
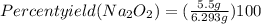
= 87.40 %
Therefore, the percentage yield of sodium peroxide is 87.4%