Answer:
The energies in descending order 35.3 eV, 28.2 eV, 18.8 eV, 16.5 eV, 11.8 eV, 7.05 eV.
Step-by-step explanation:
Given that,
Width = 0.4 nm
Number of state n= 4
We need to calculate the energy in first state
When n = 1,
Using formula of energy
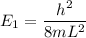
Put the value into the formula
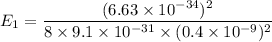
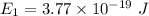
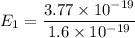
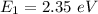
Energy in nth level is
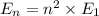
Photon energy in different states is
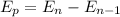
Put the value into the formula
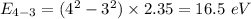
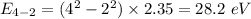
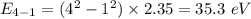
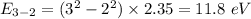
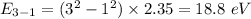
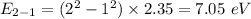
Hence, The energies in descending order 35.3 eV, 28.2 eV, 18.8 eV, 16.5 eV, 11.8 eV, 7.05 eV.