Answer : The percent yield will be, 95.06 %
Explanation : Given,
Actual yield of the reaction = 18.28 g
Expected yield of the reaction = 19.23 g
The formula used for the percent yield will be :
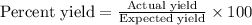
Now put all the given values in this formula, we get:
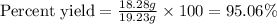
Therefore, the percent yield will be, 95.06 %