Answer : The concentration of
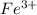 at equilibrium is 0 M.
at equilibrium is 0 M.
Solution : Given,
Concentration of
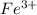 = 0.0200 M
= 0.0200 M
Concentration of
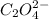 = 1.00 M
= 1.00 M
The given equilibrium reaction is,
![Fe^(3+)(aq)+3C_2O_4^(2-)(aq)\rightleftharpoons [Fe(C_2O_4)_3]^(3-)(aq)](https://img.qammunity.org/2020/formulas/chemistry/college/shqxlmoa1b81hafytykb5zi54fwam1l345.png)
Initially conc. 0.02 1.00 0
At eqm. (0.02-x) (1.00-3x) x
The expression of
 will be,
will be,
![K_c=([[Fe(C_2O_4)_3]^(3-)])/([C_2O_4^(2-)]^3[Fe^(3+)])](https://img.qammunity.org/2020/formulas/chemistry/college/kigff6zkdpdxdbv3fak2x5791xbldo1fsk.png)
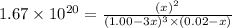
By solving the term, we get:
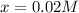
Concentration of
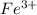 at equilibrium = 0.02 - x = 0.02 - 0.02 = 0 M
at equilibrium = 0.02 - x = 0.02 - 0.02 = 0 M
Therefore, the concentration of
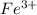 at equilibrium is 0 M.
at equilibrium is 0 M.