Answer:
93.30%
Step-by-step explanation:
The equilibrium that takes place is:
First, let's calculate the total moles of Cl⁻ added to the sample:
- 35.4 mL * 0.0219 M = 0.775 mmol Cl⁻
An unknown amount of this moles reacted with the sample, while the excess was back titrated with a solution containing Ag⁺:
- 14.33 mL * 0.0219 M *
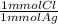 = 0.314 mmol Cl⁻
= 0.314 mmol Cl⁻
Thus, the moles of Cl⁻ that reacted with the sample are:
- 0.775 - 0.314 = 0.461 mmol Cl⁻
Then in the 0.0527 g sample there are 0.461 * 10⁻³ mol Ag, we calculate the mass of Ag in the sample:
- 0.461 * 10⁻³ mol Ag * 107.87 g/mol = 0.0497 g Ag
Finally we calculate the percent silver:
- 0.0497 g / 0.0527 g * 100% = 93.30%