Answer:
The speed of nitrogen molecule is 1.87 m/s.
Step-by-step explanation:
Given that,
Pressure = 2 atm
Density = 1.7 grams/liter
Atomic weight = 28 grams
We need to calculate the temperature
Using formula of idea gas

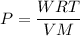
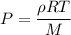
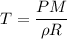
Put the value into the formula
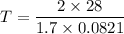
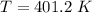
We need to calculate the speed of nitrogen molecule
Using formula of RMS speed
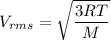
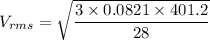
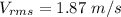
Hence, The speed of nitrogen molecule is 1.87 m/s.