Answer:
The mass of hydrogen gas in the mixture: w₂ = 0.433 g
Step-by-step explanation:
According to the ideal gas equation:
for an ideal gas,
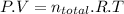
and
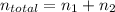
Here, P: total pressure of the gases = 2.14 atm
V: total volume of the gases = 6.68 L
T: temperature = 19 °C = 19+273.15 = 292.15K (∵ 0°C = 273.15K)
R: gas constant = 0.08206 L·atm·K⁻¹·mol⁻¹
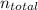 : total number of moles of gases
: total number of moles of gases
To calculate the total number of moles of gases:
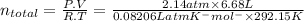 = 0.5963 moles
= 0.5963 moles
Let, the number of moles of carbon dioxide be n₁ and number of moles of hydrogen be n₂
Given: mass of carbon dioxide: w₁ = 16.8 g, mass of hydrogen: w₂ = ?g
molar mass of carbon dioxide: m₁ = 44.01 g/mol, molar mass of hydrogen: m₂= 2.016 g/mol
Therefore,
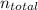 = n_{1}+n_{2} = (w₁ ÷ m₁) + (w₂ ÷ m₂)
= n_{1}+n_{2} = (w₁ ÷ m₁) + (w₂ ÷ m₂)
⇒ 0.5963 mol = (16.8 g ÷ 44.01 g/mol) + (w₂ ÷ 2.016 g/mol)
⇒ 0.5963 mol = (0.3817mol) + (w₂ ÷ 2.016 g/mol)
⇒ 0.5963 mol - 0.3817mol = (w₂ ÷ 2.016 g/mol)
⇒ 0.2146 mol = (w₂ ÷ 2.016 g/mol)
⇒ w₂ = 0.433 g
Therefore, the mass of hydrogen gas in the mixture: w₂ = 0.433 g