Answer:
The correct option is: e) n=4, l=3,
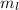 = - 2 ,
= - 2 ,
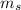 = - 1/2
= - 1/2
Step-by-step explanation:
The four quantum numbers that describe the electrons in an atom are: The principal: n, azimuthal: l, magnetic: m, and spin: s.
For a given electron, the values of the quantum numbers should be in the given range- principal quantum number: n ≥ 1;
azimuthal quantum number: 0 ≤ l ≤ n − 1;
magnetic quantum number: −l ≤
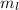 ≤ +l;
≤ +l;
spin quantum number: −s ≤
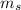 ≤ +s
≤ +s
Now, for n= 4,
The value of l: 0 to n − 1 = 0 to 3 = 0, 1, 2, 3
The value of
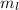 for l= 3 : -3 to +3 = -3, -2, -1, 0, +1, +2, +3
for l= 3 : -3 to +3 = -3, -2, -1, 0, +1, +2, +3
The value of
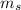 : -1/2, +1/2
: -1/2, +1/2
Therefore, the given set of quantum numbers: n=4, l=3,
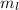 = - 2 ,
= - 2 ,
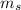 = - 1/2; are possible and permissible for an electron in an atom.
= - 1/2; are possible and permissible for an electron in an atom.