Answer:
The n = 2 → n = 3 transition results in the absorption of the highest-energy photon.
Step-by-step explanation:
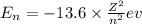
Formula used for the radius of the
 orbit will be,
orbit will be,
where,
 = energy of
= energy of
 orbit
orbit
n = number of orbit
Z = atomic number
Here: Z = 1 (hydrogen atom)
Energy of the first orbit in H atom .
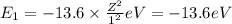
Energy of the second orbit in H atom .
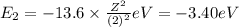
Energy of the third orbit in H atom .
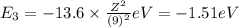
Energy of the fifth orbit in H atom .
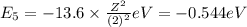
Energy of the sixth orbit in H atom .
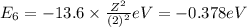
Energy of the seventh orbit in H atom .
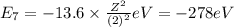
During an absorption of energy electron jumps from lower state to higher state.So, absorption will take place in :
1) n = 2 → n = 3
2) n= 5 → n = 6
Energy absorbed when: n = 2 → n = 3
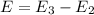
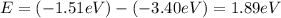
Energy absorbed when: n = 5 → n = 6
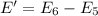
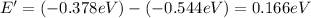
1.89 eV > 0.166 eV
E> E'
So,the n = 2 → n = 3 transition results in the absorption of the highest-energy photon.