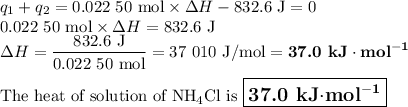Answer:
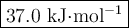
Step-by-step explanation:
There are two heat transfers involved in this problem.
Heat of solution of AlCl₃ + heat lost by water = 0
q₁ + q₂ = 0
nΔH + mCΔT = 0
Let's calculate the heats separately.
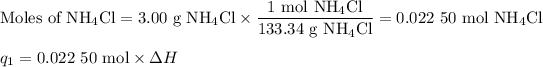
m = 100.00 g
C = 4.18 J·°C⁻¹g⁻¹
ΔT = -1.99 °C
q₂ = mCΔT = 100.00 g × 4.184 J·°C⁻¹g⁻¹ × (-1.99 °C) = -832.6 J