Answer:
A. Molarity of the Ba(OH)₂ solution is 0.128 mol/L or 0.128 M
B. Molarity of the HClO₄ solution is 0.653 mol/L or 0.653 M.
Step-by-step explanation:
A) Given: Given mass of Ba(OH)₂: w= 1.34 g, Molar mass of Ba(OH)₂: m= 171.34 g/mol, Volume of the solution: V = 61.1 mL
The molarity of Ba(OH)₂:
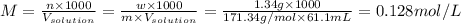
Therefore, the molarity of the Ba(OH)₂ solution is 0.128 mol/L or 0.128 M
B) The neutralization reaction:
Ba(OH)₂ (aq) + 2 HClO₄(aq) → Ba(ClO₄)₂ (aq) + 2 H₂O(l)
Volume of Ba(OH)₂: V₁ = 15.1 mL ; volume of HClO₄: V₂ = 5.92 mL
Concentration of Ba(OH)₂: M₁ =
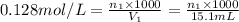
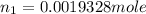
Now, concentration of HClO₄: M₂= ?
As 1 mole of barium hydroxide neutralizes 2 moles of perchloric acid.
∴ number of moles of HClO₄: n₂= 2 × number of moles of Ba(OH)₂ = 2 \times 0.0019328 mole = 0.00386 moles
Now, the molarity of HClO₄:
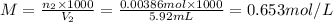
Therefore, the molarity of the HClO₄ solution is 0.653 mol/L or 0.653 M.