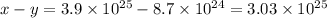Answer:
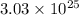 particles of
particles of
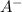 are in the container after the neutralization.
are in the container after the neutralization.
Step-by-step explanation:
Chemical equation for the neutralization reaction:

Number of particles of HA dissolved in water ,x=
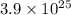
Single molecule of HA had single
 and 1
and 1
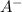 .
.
So , Number of particles of
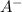 dissolved in water = x
dissolved in water = x
Number of particles HA which get neutralized by adding NaOH : y
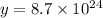
Number of
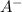 particles get neutralized by adding NaOH = y
particles get neutralized by adding NaOH = y
Number of
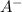 particles that are in the container after the neutralization:
particles that are in the container after the neutralization: