Answer:
The volume % of SO₂ in the original sample is 1,59%
Step-by-step explanation:
For the reaction:
2SO₂(g) + I₂(aq) + 2H₂O(l) → 2HSO4¹⁻(aq) + 2I¹⁻(aq) +4H⁺(aq)
The add moles of iodine are:
0,0200L×
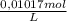 = 2,034x10⁻⁴ moles of I₂
= 2,034x10⁻⁴ moles of I₂
The moles of thiosulfate for the reaction:
I₂(aq) + 2S₂O₃²⁻(aq) → 2I⁻(aq) + S₄O₆²⁻(aq)
are:
0,01137L×
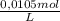 = 1,194x10⁻⁴ moles of S₂O₃²⁻
= 1,194x10⁻⁴ moles of S₂O₃²⁻
Thus, excess moles of I₂ are:
1,194x10⁻⁴ moles of S₂O₃²⁻×
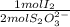 = 5,969x10⁻⁵ moles of I₂
= 5,969x10⁻⁵ moles of I₂
That means that moles of I₂ that react with sulfur dioxide are:
2,034x10⁻⁴ moles of I₂ - 5,969x10⁻⁵ moles of I₂ = 1,4371x10⁻⁴ moles of I₂
These moles of I₂ are:
1,4371x10⁻⁴ moles of I₂×
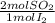 = 2,8742x10⁻⁴ moles of SO₂
= 2,8742x10⁻⁴ moles of SO₂
Using:
V = nRT/P
Where n are moles (2,8742x10⁻⁴ moles of SO₂)
R is gas constant (0,082atmL/molK)
T is temperature (38°C ≡ 311,15K)
P is pressure (700torr ≡ 0,921 atm)
The volume that SO₂ occupy is:
V = 7,962x10⁻³L ≡ 7,962mL
Thus, volume % is:
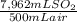 ×100 = 1,59 Volume%
×100 = 1,59 Volume%
I hope it helps!