Answer:
a. 28,7 mL
b. pH = 2,16
c. pH = 4,09
d. pH = 1,01
Step-by-step explanation:
a. The titration of sodium benzoate with nitric acid is:
C₆H₆COONa + HNO₃ → C₆H₆COOH + NaNO₃
The moles of sodium benzoate (NaBz) are:
0,0200L ×
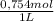 = 0,01508mol of NaBz
= 0,01508mol of NaBz
As molar ratio in the titration is 1:1, moles of nitric acid you need to add at the equivalence point are 0,01508mol, in mL:
0,01508mol×
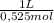 = 0,0287L = 28,7 mL
= 0,0287L = 28,7 mL
b. In equivalence point, all 0,754M of NaBz were converted in benzoic acid.
As acidic equilibrium of benzoic acid (Bz) is:
C₆H₆COOH ⇄ C₆H₆COO⁻ + H⁺; pka = 4,20; ka = 10^-4,20; ka = [C₆H₆COO⁻][H⁺]/[C₆H₆COOH]
In equilibrium:
[C₆H₆COOH] = 0,754-x
[C₆H₆COO⁻] = x
[H⁺] = x;
Replacing in ka:
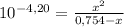
4,757x10⁻⁵ - 6,31x10⁻⁵x = x²
x² + 6,31x10⁻⁵x - 4,757x10⁻⁵ = 0
Solving for x:
x = -0.00692872 No physical sense.
x = 0.00686562 Real answer.
As pH = -log [H⁺]; pH = 2,16
c. The moles of nitric acid in 16,20mL are:
0,01620L×0,525M = 8,505x10⁻³ moles, that are the same moles of benzoic acid. Thus, moles of NaBz are:
0,01508mol - 8,505x10⁻³ moles = 6,575x10⁻³ moles
Using Henderson-Hasselbalch formula:
pH = pka + log [C₆H₆COONa] / [C₆H₆COOH]
pH = 4,20 + log 6,575x10⁻³/8,505x10⁻³
pH = 4,09
d. After addition of 28,7mL of nitric acid, the additional volume will produce free H⁺. Additional volume is: 39,82mL - 28,7mL = 11,12mL. In moles of HNO₃ ≡ moles of H⁺:
0,01112L×
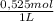 = 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:
= 5,838x10⁻³moles of H⁺. The volume is 20,0mL + 39,82mL = 59,82mL ≡ 0,05982L. Thus, [H⁺] is:
5,838x10⁻³moles / 0,05982L = 0,09759 M. pH = -log [0,09759] = 1,01
I hope it helps!