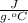Answer:
The specific heat of water is Cp = 4.184
Step-by-step explanation:
1. The specific heat can be expressed as:
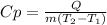
where Q is the energy, m is the mass of water, and T2-T1 express the change in the temperature.
2. Replace values for mass, energy and temperature:
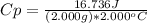
3. Solve the equation to obtain the specific heat of water:
Cp=4.184