Answer:
0.0295 liters is the minimum amount of the NaOH(aq) solution we would add.
Step-by-step explanation:
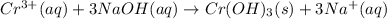
Molarity of chromium ions ,M= 0.0130 M
Volume of chromium ions ,V= 0.600 L
Moles of chromium ions = n
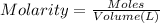
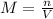
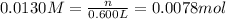
According to reaction , 1 moles of chromium ions reacts with 3 moles of NaOH.
Then 0.0078 moles of chromium ions will react with :
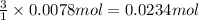 of NaOH
of NaOH
Moles of NaOH = n' = 0.0234 moles
Molarity of NaOH solution = M'= 1.26 M
Volume of the NaOH solution = V' = ?
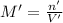
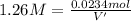
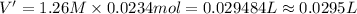
0.0295 liters is the minimum amount of the NaOH(aq) solution we would add.